What Causes Corrosion
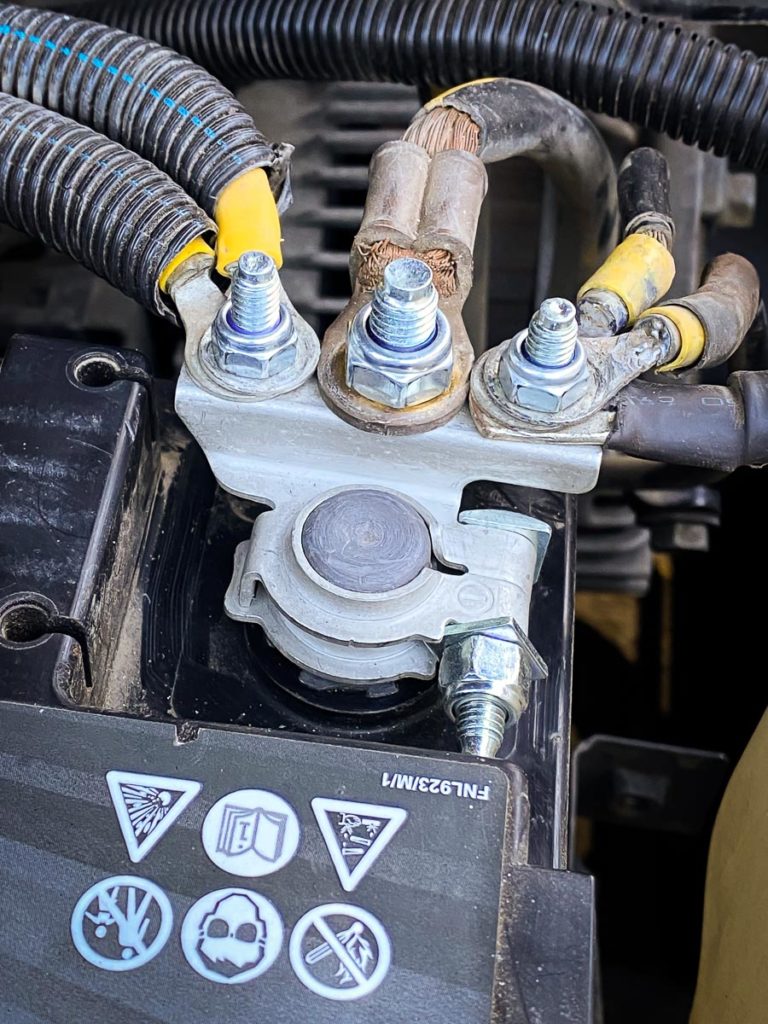
Battery terminal corrosion is commonly seen as a white-blue or greenish powder that gathers around the positive- and negative-battery posts, well our CMH Renault Midrand Workshop Technicians suggest that corrosion is generally caused by hydrogen gas (which is released from the battery while charging), along with moisture and other salts / minerals in the atmosphere, all of which combine to create a corrosive environment. In many cases, it’s believed that the corrosion will typically form around the negative terminal, which suggests that the battery is “undercharging” due to a lack of adequate driving time. Corrosion around the positive terminal is generally believed to be the result of overcharging from the alternator. Either way, any form of battery terminal corrosion will eventually lead to poor conductivity, high electrical resistance, reduced lifespan, and ultimately the inability to deliver enough energy to start the engine.Overcharging
If your alternator is slightly overcharging your car battery, it might cause corrosion on your car battery terminals. Check your voltage with a multi-meter when your car runs to make sure it is not charging over 14.5 volts when you are revving the engine. It can also be because you are frequently charging your car battery with a car battery charger too hard.Overfilling the battery
If you overfill your car battery, it might cause the electrolyte to leak out, as mentioned before. Not all car batteries are refillable, but you should absolutely double-check if you have one so it is not overfilled.Hydrogen Gas Leakage
The battery turns acid into an electric current. There are moments that the hydrogen gas in the battery leaks and finds its way into the atmosphere. It reacts with other substances, and you get battery terminal corrosion. Depending on which side it forms, you can diagnose various battery problems. If it is on the negative terminal, this is a sign of undercharging, while if it is on the positive terminal, it is due to overcharging.How To Prevent Battery Corrosion
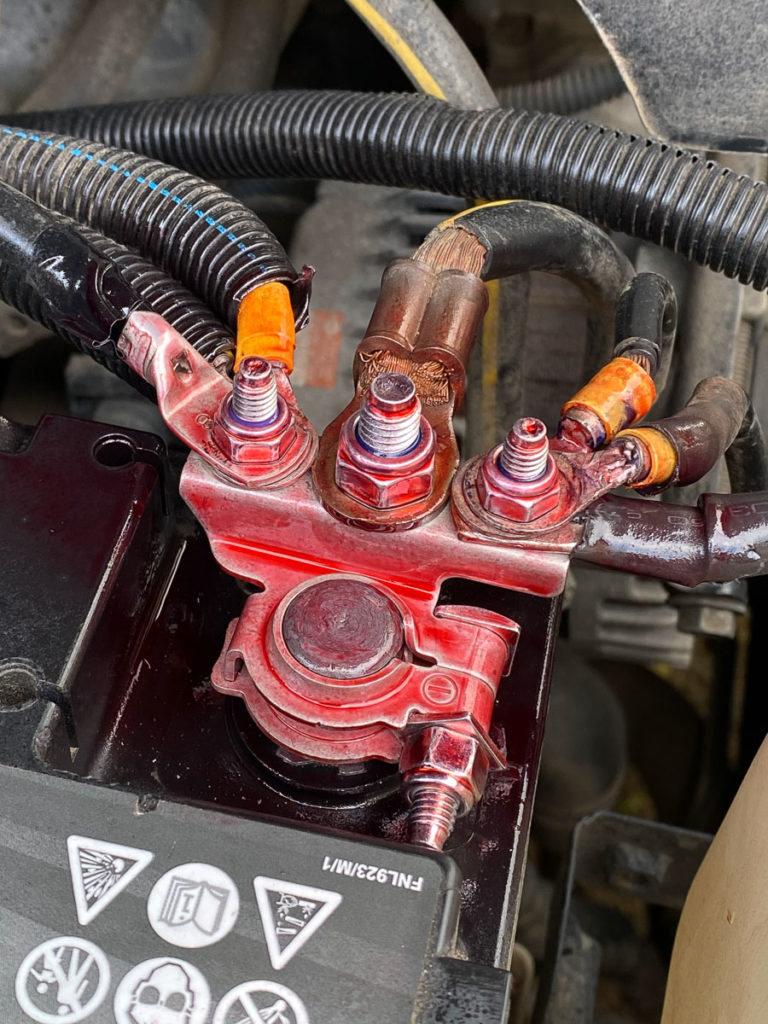
Although some mechanics use grease or Vaseline to cover the battery’s terminals, our amazing of team of experts at our CMH Renault Workshop can guarantee you that these substances attract dirt. Needless to say, a gradual build-up of dirt and dust can also contribute to poor electrical contact. According to CRC Industries RSA (Pty) Ltd – trading as CRC & Q20 South Africa the correct product to use is CRC’S BATTERY TERMINAL PROTECTOR, which is one of a lead-free soft coating that’s specifically designed to protect battery terminals from corrosion.CRC’S BATTERY TERMINAL PROTECTOR does the following:
- Prolong battery life
- Guarantee successful charging and maintenance
- Assure easy starting
- Prevent corrosion
How To Apply:
- Shake can well before use
- Clean away existing corrosion with it/li>
- Apply an even coat to freshly cleaned hold-downs, bolts, brackets and terminals/li>
- For preventative maintenance: reapply when the red film turns clear/li>